Miniature brains that show electrical activity akin to “a primitive type of thinking” could revolutionise how some drugs are tested and reduce the need for animals in research, according to scientists who have developed the structures.
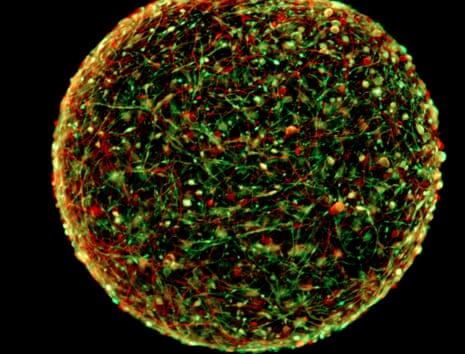
Each ball of human brain cells - in all about the size of the head of a ballpoint pen - “represents more or less a two-month-old brain” of a foetus, Prof Thomas Hartung of Johns Hopkins University said, presenting the work at the American Association for the Advancement of Science conference . The cultures also show “spontaneous electrophysiological activity” – their neurons zap off electrical signals to each other without prompting.
“It’s starting to produce a primitive type of ‘thinking,’” Hartung said, though he stressed that the operation is only mechanical. “Obviously there’s no input or output. It is meaningless electrical activity but the neurons are trying to communicate with each other.”
Scientists first developed miniature brains in test tubes in December 2013, but Hartung said that his team has managed to standardise the new “mini-brains” – meaning they can grow hundreds of uniform brain cultures in a single petri dish, rather than a few cultures each grown according to their distinct genetic codes.
Harvard professor Rudolph Tanzi, who in 2014 developed human brain cells in a gel as part of his work on Alzheimer’s, told the Guardian that he and his colleagues had also seen primitive neural activity.
“They’re doing something very similar, even the types of cells they’re seeing is similar to what we’re seeing,” Tanzi said, adding that he questioned whether the new brains are more “standardised” than others. “But it’s great, great to see that the technology is catching on. I think the more labs that do this the better.”
Hartung said the neural activity, which can be measured with an EEG-like device, creates a “third dimension” for studying the effects of drugs: not only can researchers examine how drugs affect the health and function of brain cells, they can also see what drugs do to neural activity.
Tanzi agreed, saying that in the brain cells with Alzheimer’s disease “the signalling is much different. You get plaques and tangles that become dysfunctional,” referring to protein clusters and cell transport systems that the disease affects.
The Johns Hopkins mini-brains appear to be capable of such activity because of their relatively mature development: by developing in dense clusters, according to Hartung, the brains “build these circuits of stimulating each other”, as well as the six types of neurons and accessory cells in brains.
“It is very difficult to produce these type of stem cell cultures artificially, but they’re wonderfully forming because they’re not alone here,” he said. “This brain model differs from others in that we spent a lot of effort into the standardisation and quality control.”
The new brains are smaller than older models, and can only develop as far as researchers provide oxygen, nutrients and petri dish or test tube space to allow them. Larger brain models also have development limits and eventually the internal neurons die, since they lack the structure of a body – namely a blood supply to their center – that would support them.
The Johns Hopkins team grew the brains from five donors’ skin cells, which were reprogrammed to become the equivalent of stem cells, and then stimulated toward becoming brain cells. The published research features cells recreated with conditions of Parkinson’s disease, which is believed to develop out of a mix of genetic and environmental factors.
The team hopes that the mini-brains will help studies on Parkinson’s, Alzheimer’s, multiple sclerosis (MS) and autism – each neurological conditions caused by both genetic and environmental factors.
Hartung has applied for a patent on the mini-brains and is developing a company to manufacture the cells. Tanzi and his colleagues opted for an “open-sourced” method. “Our goal is to spread this out throughout the world,” he said.
The cultures could also help phase out the use of animals for testing drugs and treatments – Hartung is also head of Johns Hopkins’ Center for Alternatives to Animal Testing. “The animal models are misleading,” he said.
Tanzi agreed with Hartung’s assertion that animal testing is unreliable. “A human is not a 150lb rat, or a 150lb mouse,” Tanzi said. Hartung added: “And even though we are not balls of cells either, you can often get much better information from these balls of cells.”
Comments (…)
Sign in or create your Guardian account to join the discussion